0023-6146 : Fiorinal Oral Capsule
| NDC: | 0023-6146 |
| Labeler: | Allergan, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Fiorinal Fiorinal |
| Dosage Form: | Oral Capsule |
| Application #: | NDA017534 |
| Rev. Date: | |
| CSA Schedule: | CIII (US) [1] |
[1] Schedule III / IIIN Controlled Substance: Has a potential for abuse less than substances in Schedules I or II and abuse may lead to moderate or low physical dependence or high psychological dependence. (i.e. Products containing not more than 90 milligrams of Codeine per dosage unit [such as Tylenol with Codeine], other narcotics such as Buprenorphine (Suboxone), and Schedule IIIN non-narcotics such as Didrex, Ketamine, Phendimetrazine, and Anabolic Steroids). More Details: US Dept of Justice Controlled Substance Schedules.
Appearance:
| Markings: | FIORINAL;955 |
| Shapes: |
Capsule |
| Colors: |
 Green Green |
| Size (mm): | 29 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
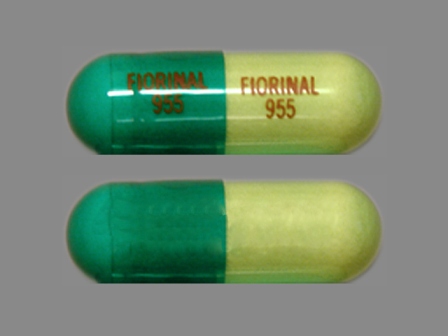
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0023-6146-01: 100 CAPSULE IN 1 BOTTLE (0023‑6146‑01)
Active Ingredients:
- Butalbital
- Aspirin
- Caffeine
Dosage Strength:
- 50 mg
- 325 mg
- 40 mg
Inactive Ingredients:
- Cellulose, Microcrystalline
- Starch, Corn
- Talc
- D&c Yellow No. 10
- Fd&c Green No. 3
- Gelatin
- Ferric Oxide Red /
Pharmaceutical Classes:
- Barbiturates [CS]
- Barbiturate [EPC]
- Platelet Aggregation Inhibitor [EPC]
- Decreased Platelet Aggregation [PE]
- Cyclooxygenase Inhibitors [MoA]
- Decreased Prostaglandin Production [PE]
- Anti-Inflammatory Agents
- Non-Steroidal [CS]
- Nonsteroidal Anti-inflammatory Drug [EPC]
- Central Nervous System Stimulant [EPC]
- Methylxanthine [EPC]
- Xanthines [CS]
- Central Nervous System Stimulation [PE]
Related Products:
Based on records with the same trade name.- 52544-955 Fiorinal Oral Capsule by Watson Pharma, Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0023-6145Next: 0023-6147 >
Related Discussions:
Fiorinal (aspirin, butalbital, caffeine)
Information on uses, side effects, dosing, mechanism of action and other pharmacology. ## I am looking for information o... 3 replies
Information on uses, side effects, dosing, mechanism of action and other pharmacology. ## I am looking for information o... 3 replies
Butalbital 50 + Aspirin 325 + Caffeine 40
My boyfriend is a Vietnam Vet and he has a rod in his leg and suffers from migraines all the time sometimes 3 days strai... 2 replies
My boyfriend is a Vietnam Vet and he has a rod in his leg and suffers from migraines all the time sometimes 3 days strai... 2 replies
replacement for butalbital aspirin caffeine 50/325/50?
Is there a replacement for butalbital/aspirin/caffeine out there 50/325/50? It was the combo of the drug that worked for... 5 replies
Is there a replacement for butalbital/aspirin/caffeine out there 50/325/50? It was the combo of the drug that worked for... 5 replies
butalbitalapapcaffeine 5032540
Gen Eq: FIORICET, what is this medication and how does it make you feel and what is it for. will it cause anexity? ## I ... 5 replies
Gen Eq: FIORICET, what is this medication and how does it make you feel and what is it for. will it cause anexity? ## I ... 5 replies
Butalbital/Apap/Caffeine Tablets
Butalbital/Apap/Caffeine Tablets for tension headaches ## For more info on this drug check out the page on Acetaminophen... 13 replies
Butalbital/Apap/Caffeine Tablets for tension headaches ## For more info on this drug check out the page on Acetaminophen... 13 replies
Butalbital Apap Caffeine with Tylenol
I am pregnant and I'm taking butalbital + apap (325mg) + caffeine. My doctor says it's ok. Can I take tylenol al... 2 replies
I am pregnant and I'm taking butalbital + apap (325mg) + caffeine. My doctor says it's ok. Can I take tylenol al... 2 replies
butalbital acetaminophen caffeine 50/325/40 uses
How long does it take for the pain from a bad migraine to go away? I've been taking the pills for 3 days and I still... 7 replies
How long does it take for the pain from a bad migraine to go away? I've been taking the pills for 3 days and I still... 7 replies
butalbital acetaminophen caffeine and codeine
blue and tan capsule westward 3000 ## This is a generic version of Fiorcet, most often used for severe headaches and mig... 3 replies
blue and tan capsule westward 3000 ## This is a generic version of Fiorcet, most often used for severe headaches and mig... 3 replies
butalbital + acetaminophen + caffeine 50/325/40
There is no label on my butalbital + acetaminophen + caffeine. What is it used for? And is it a narcotic? ## Hello, Patr... 1 reply
There is no label on my butalbital + acetaminophen + caffeine. What is it used for? And is it a narcotic? ## Hello, Patr... 1 reply
Acetaminophen 325mg + Butalbital 50mg + Caffeine 40mg availability
I would like to know if these are available online, either in the UI, SA, A, or Canada..? Any information, address, tele... 2 replies
I would like to know if these are available online, either in the UI, SA, A, or Canada..? Any information, address, tele... 2 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.