0054-8650 : Roxicet 5/325 Oral Tablet
| NDC: | 0054-8650 |
| Labeler: | Roxane Laboratories, Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Roxicet Roxicet |
| Dosage Form: | Oral Tablet |
| Application #: | ANDA087003 |
| Rev. Date: | |
| CSA Schedule: | CII (US) [1] |
[1] Schedule II / IIN Controlled Substance: High potential for abuse which may lead to severe psychological or physical dependence. (i.e. Narcotics such as Dilaudid, Methadone, Demerol, Oxycodone, Percocet, Fentanyl, Morphine, Opium, Codeine, and Hydrocodone ... Schedule IIN stimulants include non-narcotic Amphetamines such as Dexedrine, Adderall, Desoxyn, Methylphenidate (Ritalin) ... Other Schedule II substances include Amobarbital, Glutethimide, and Pentobarbital. More Details: US Dept of Justice Controlled Substance Schedules.
Appearance:
| Markings: | 54;543 |
| Shapes: |
Round |
| Colors: |
 White White |
| Size (mm): | 11 |
| Segments: * | 2 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 2 indicates a scored pill which can be broken into 2 equal pieces. | |
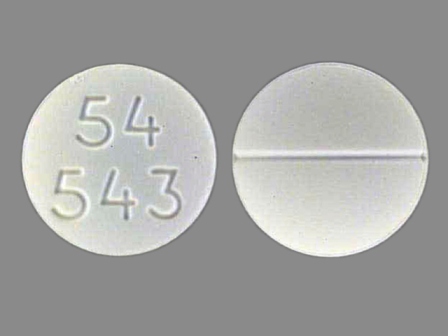
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0054-8650-24: 4 BLISTER PACK IN 1 BOX (0054‑8650‑24) > 25 TABLET IN 1 BLISTER PACK
Active Ingredients:
- Oxycodone Hydrochloride
- Acetaminophen
Dosage Strength:
- 5 mg
- 325 mg
Inactive Ingredients:
- Silicon Dioxide
- Croscarmellose Sodium
- Cellulose, Microcrystalline
- Stearic Acid
Pharmaceutical Classes:
- Full Opioid Agonists [MoA]
- Opioid Agonist [EPC]
Related Products:
Based on records with the same trade name.- 0054-3686 Roxicet Oral Solution by Roxane Laboratories, Inc.
- 0054-4650 Roxicet 5/325 Oral Tablet by Roxane Laboratories, Inc.
- 0054-4784 Roxicet 5/500 Oral Tablet by Roxane Laboratories, Inc.
- 0054-8648 Roxicet Oral Solution by Roxane Laboratories, Inc.
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0054-8648Next: 0054-8722 >
Related Discussions:
oxycodone acetaminophen 10 325 changes
I HAVE OA AND need both knees and right hip replaced, I also have bone infarcts in both legs and in 2003 had a car accid... 8 replies
I HAVE OA AND need both knees and right hip replaced, I also have bone infarcts in both legs and in 2003 had a car accid... 8 replies
oxycodone acetaminophen 10 325 mg What is the time limit for the drug store to fill this prescription.
Oxycodone Acetamnophen 10 325MG TB What is the time limited for the drug store to fill this prescription. ## Hello, Ann!... 6 replies
Oxycodone Acetamnophen 10 325MG TB What is the time limited for the drug store to fill this prescription. ## Hello, Ann!... 6 replies
Oxycodone Acetaminophen 5 325 and still in pain
I had a total knee replacement done last Monday. When released on Thursday, I was prescribed with Oxycodone Acetaminophe... 5 replies
I had a total knee replacement done last Monday. When released on Thursday, I was prescribed with Oxycodone Acetaminophe... 5 replies
oxycodone acetaminophen7. 5/ 325 tab all brands reactions
I need to know about this medicine. I have been taking Oxycodone-APAP 7.5/325 mg (Mallin). Last month the pharmacy chang... 4 replies
I need to know about this medicine. I have been taking Oxycodone-APAP 7.5/325 mg (Mallin). Last month the pharmacy chang... 4 replies
Oxycodone Acetaminophen 7 5 325
What does the oxycodone-acetaminophe 7.5-325 tab manufactured by alvogen look like? The one I have is round and peach in... 4 replies
What does the oxycodone-acetaminophe 7.5-325 tab manufactured by alvogen look like? The one I have is round and peach in... 4 replies
oxycodone acetaminophen shortage? in severe pain
I'm on pain management for 3 bulging now possibly herniated discs(I have to get a new MRI and EMG This week) and a k... 4 replies
I'm on pain management for 3 bulging now possibly herniated discs(I have to get a new MRI and EMG This week) and a k... 4 replies
Oxycodone acetaminophen 5/325 affect breathing?
Does this affect your breathing and what is the 5 mixture? ## Hello, K! How are you? This is 5mgs of Oxycodone coupled w... 3 replies
Does this affect your breathing and what is the 5 mixture? ## Hello, K! How are you? This is 5mgs of Oxycodone coupled w... 3 replies
oxycodone acetaminophen 5 325mg does it contain aspirin
I would like to know if aspirin is in oxycodone. My husband has back pain and I have oxycodone/acetaminophen5-325 I use ... 3 replies
I would like to know if aspirin is in oxycodone. My husband has back pain and I have oxycodone/acetaminophen5-325 I use ... 3 replies
oxycodone acetaminophen 10 325 mg /hydrocodone 10-325
I was taking hydrocodon 10-325 and now my dr switched me to Percocet 10-325 and when I take it it puts me to sleep for h... 2 replies
I was taking hydrocodon 10-325 and now my dr switched me to Percocet 10-325 and when I take it it puts me to sleep for h... 2 replies
Oxycodone Acetaminophen 10 325 6x a day
I have been on Oxycodone 10/325 for over three years, taking 6 per day. My doctor wants to change what would be comparab... 2 replies
I have been on Oxycodone 10/325 for over three years, taking 6 per day. My doctor wants to change what would be comparab... 2 replies
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.